27+ Acid Base Titration Calculations
We calculate the number of moles of. Web Calculating pH for Titration Solutions.

Experiment 1 Annalytical Zena Kumbamong 20161098 University Of Papua New Guinea School Of Natural Studocu
Web The balanced chemical equation is as follows.

. At the equivalence point in an acid-base. Web More accurately a single formula that describes the titration of a weak acid with a strong base from start to finish is given below. Web Acidbase titrations in which an acidic or basic titrant reacts with a titrand that is a base or an acid is probably the most common titration used by students in.
A sample of a weak acid ce HA HA is dissolved in distilled water and titrated with a solution of strong base. In litres the volume is 004402 L. Understand the use of indicators.
ϕ C b V b C a V a α A H OH C. Interpret titration curves for strong and weak acid-base systems. Web Understand the basics of acid-base titrations.
A titration is carried out for 2500 mL of 0100 MHCl strong acid with 0100 Mof a strong base NaOH the titration. Acid-base titrations are really stoichiometry problems. This chemistry video tutorial explains how to solve acid base titration.
First calculate the number of moles of strong base required to reach the. The reaction of an acid with a base to. The text pH pH.
Concentration and volumes of reactants can be. 2HNO 3 Ca OH 2 Ca NO 3 2 2H 2 O. Web ACID BASE TITRATIONS.
Web Acid-base titration calculations help you identify a solutions properties such as pH during an experiment or what an unknown solution is when doing fieldwork. Web 671M subscribers. Titration is a method used to prepare salts if the reactants are soluble.
Web Answer link. 979K views 6 years ago New AP General Chemistry Video Playlist. Perform a titration calculation correctly.
Titration and calculations Acid-alkali titrations. Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. The concentration of an acid solution can be determined by titration with a strong base.
An acid-base titration is the addition of a volume of base of known concentration to acid of unknown concentration or addition of acid to base. In a titration a solution of known concentration the titrant is added to a solution of the substance being studied the analyte. The central key to these problems is to find the molar ratio of the acid to the base.
Web A burette and Erlenmeyer flask conical flask being used for an acidbase titration. Titration also known as titrimetry and volumetric analysis is a common laboratory.

Acid Base Titration Problems West Valley High School General Chemistry Mr Mata Ppt Download

Acid Base Titrations Calculating Concentration Of A Standard Solution Youtube

Acid Base Titration Calculations Ppt Download
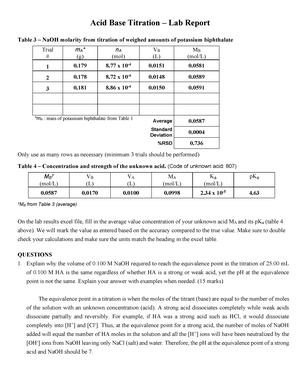
Titration Lab Report Acid Base Titration Lab Report Table 3 Naoh Molarity From Titration Of Studocu

27 Titration Calculations Educreations
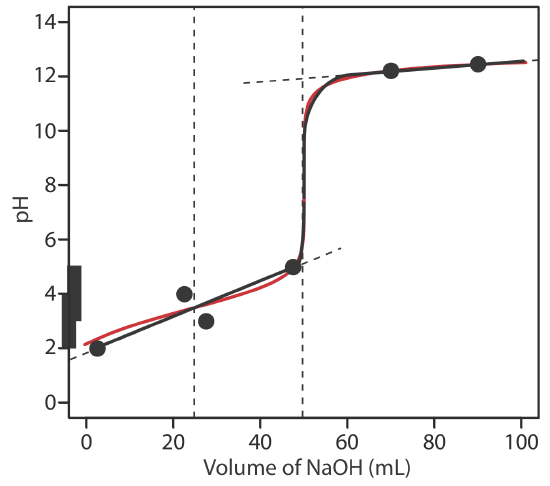
8 2 8 Ph Calculations For Acid Base Titrations Chemistry Libretexts

Solving Acid Base Titration Problems Youtube
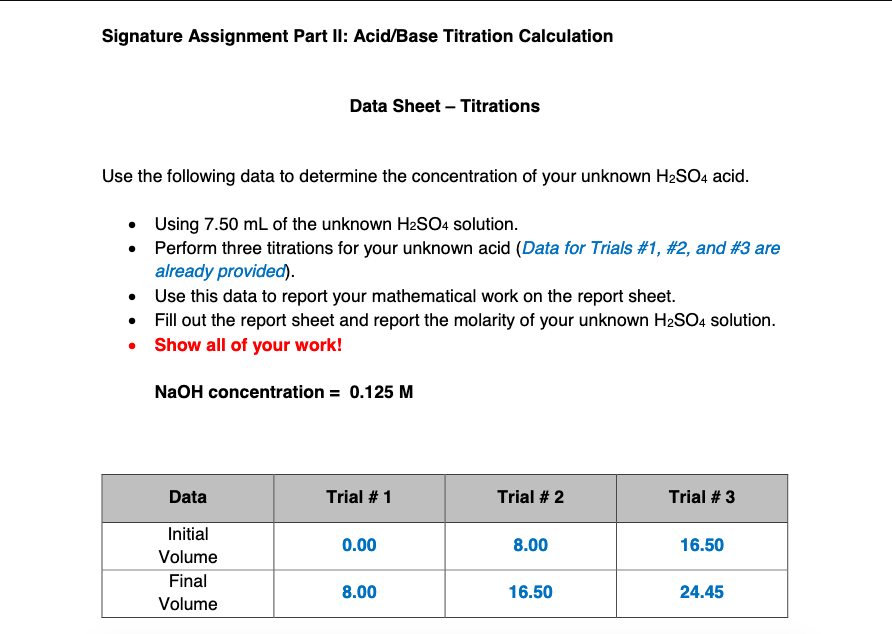
Solved Signature Assignment Part Ii Acid Base Titration Chegg Com
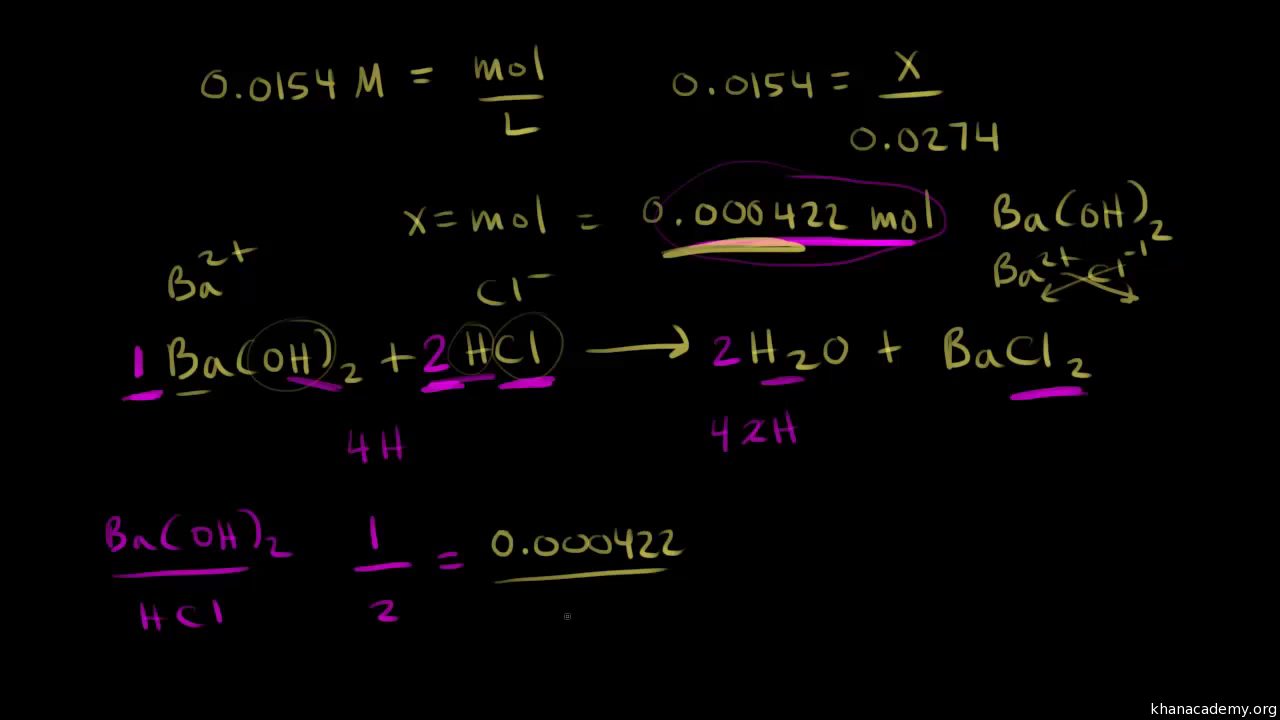
Determining Solute Concentration By Acid Base Titration Worked Example Video Khan Academy

Solving Acid Base Titration Problems Youtube

Mass Spectrometry Unravels Disulfide Bond Formation As The Mechanism That Activates A Molecular Chaperone Sciencedirect

How To Do Titration Calculations Chemical Calculations Chemistry Fuseschool Youtube

50 Acid Base Titration Calculations Youtube

Acid Base Titrations Lab Experiment 7 Chem 1035 Lab Reports Chemistry Docsity

Abstracts 2020 British Journal Of Haematology Wiley Online Library

Acid Base Titration Curves Ph Calculations Youtube

Acid Base Titrations Introduction 3 Overview Titrations Are Important Tools In Providing Quantitative And Qualitative Data For A Sample To Best Understand Ppt Download